Cancer Res.:肿瘤细胞遭溶瘤病毒感染后发出清除病毒警报
2012-03-23 MedSci MedSci原创
根据来自美国俄亥俄州立大学综合癌症中心–Arthur G. James肿瘤医院和Richard J. Solove研究所(The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital and Richard J. Solove Research Institute)的研究人员开展的一项
根据来自美国俄亥俄州立大学综合癌症中心–Arthur G. James肿瘤医院和Richard J. Solove研究所(The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital and Richard J. Solove Research Institute)的研究人员开展的一项研究,感染着肿瘤杀手病毒(tumor-killing virus)的脑瘤细胞释放一种起着“警铃(alarm bell)”作用的蛋白来通知其他的肿瘤细胞即将来临的病毒感染,并能够让它们发动对病毒的抵抗。相关研究结果于2012年3月15日发表在Cancer Research期刊上。
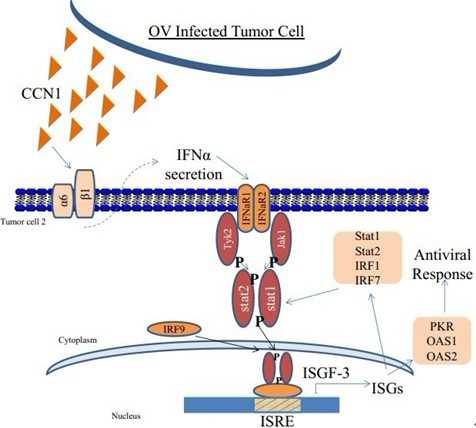
这些被感染的肿瘤细胞将一种被称作CCN1(Cysteine rich 61, 译者注:也称作CYR61)的蛋白释放进细胞之间的狭窄空间中,而且在那里这种蛋白启动抗病毒反应。这种反应限制溶瘤病毒(oncolytic virus)在肿瘤内传播的速度,降低它杀死癌细胞的能力,从而限制利用这种病毒进行抗肿瘤治疗的效果。
这项研究提示着肿瘤细胞一般来说可能能够利用这种机制来有助于控制病毒复制,同时也提示着阻断这一反应很可能改善治疗神经胶质瘤(glioblastoma)的溶瘤病毒疗法,并可能改善未来的基因疗法。
溶瘤病毒在肿瘤细胞中复制并杀死它们。已有实验表明这种病毒有望用于治疗神经胶质瘤---脑癌中最为常见和最为致命性的形式。患有神经胶质瘤的病人在确诊之后平均只能存活大约15个月,所以人们急需要一种新的疗法。
“我们发现在胞外基质,这种蛋白精心安排一种显著性的细胞抗病毒反应来降低病毒复制并限制它的溶细胞效应”,主要研究员Balveen Kaur副教授说。
“这些发现是比较有意义的,因为它们揭示出被病毒感染的肿瘤细胞利用一种新机制来对抗病毒感染,并发出警报通知周围未被感染的肿瘤细胞做好防御以便击退即将到来的病毒攻击”,Kaur说。
Kaur注意到,CCN1有助于调节包括附着、迁移和增殖在内的细胞功能,而且在68%的神经胶质瘤样品中它是过表达的。
Kaur在以前的研究中就已发现溶瘤病毒疗法诱导肿瘤细胞释放CCN1到肿瘤微环境中。在这项研究中,Kaur和她的同事们使用神经胶质瘤细胞系、来自人疱疹病毒I型(human herpesvirus type 1, HSV-1)的溶瘤病毒和神经胶质瘤模式动物来开展研究。主要研究结果包括:
1)溶瘤病毒上调CCN1表达,但是化疗或放射治疗不能如此。因此,它可能是神经胶质瘤细胞对病毒感染产生的一般对策。
2)在胞外空间,CCN1降低病毒复制从而阻止它杀死神经胶质瘤细胞。
3)CCN1通过一种整合素细胞表面受体(integrin cell-surface receptor)来诱导I型干扰素抗病毒反应。
“总体而言,这项发现揭示胞外信号传导如何能够促进肿瘤细胞清除病毒”,Kaur说,“我们如今能够利用这种知识来改善未来的抗病毒基因治疗。” (生物谷:towersimper编译)

doi:10.1158/0008-5472.CAN-11-2526
Extracellular Matrix Protein CCN1 Limits Oncolytic Efficacy in Glioma
Amy Haseley, Sean Boone, Jeffrey Wojton, Lianbo Yu, Ji Young Yoo, Jianhua Yu, Kazuhiko Kurozumi, Joseph C. Glorioso, Michael A. Caligiuri, and Balveen Kaur
Oncolytic viral therapy has been explored widely as an option for glioma treatment but its effectiveness has remained limited. Cysteine rich 61 (CCN1) is an extracellular matrix (ECM) protein elevated in cancer cells that modulates their adhesion and migration by binding cell surface receptors. In this study, we examined a hypothesized role for CCN1 in limiting the efficacy of oncolytic viral therapy for glioma, based on evidence of CCN1 induction that occurs in this setting. Strikingly, we found that exogenous CCN1 in glioma ECM orchestrated a cellular antiviral response that reduced viral replication and limited cytolytic efficacy. Gene expression profiling and real-time PCR analysis revealed a significant induction of type-I interferon responsive genes in response to CCN1 exposure. This induction was accompanied by activation of the Jak/Stat signaling pathway, consistent with induction of an innate antiviral cellular response. Both effects were mediated by the binding of CCN1 to the cell surface integrin α6β1, activating its signaling and leading to rapid secretion of interferon-α, which was essential for the innate antiviral effect. Together, our findings reveal how an integrin signaling pathway mediates activation of a type-I antiviral interferon response that can limit the efficacy of oncolytic viral therapy. Furthermore, they suggest therapeutic interventions to inhibit CCN1–integrin α6 interactions to sensitize gliomas to viral oncolysis.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言








#Res.:#
70
#肿瘤细胞#
66