SCTM:首次开发出生产成体干细胞的方法
2012-02-15 towersimper 生物谷
澳大利亚昆士兰大学科学家在世界上首次开发出生产成体干细胞的方法,这一研究成果将深刻影响着患有一系列严重性疾病的病人。 这项研究是包括昆士兰大学澳大利亚生物工程和纳米技术研究所在内的多家研究机构合作开展的,由昆士兰大学临床研究中心教授Nicholas Fisk领导。 间充质干细胞(mesenchymal stem cells, MSCs)能够被用来修复骨骼和潜在性地修复其他器官。这项研究揭示一种
澳大利亚昆士兰大学科学家在世界上首次开发出生产成体干细胞的方法,这一研究成果将深刻影响着患有一系列严重性疾病的病人。
这项研究是包括昆士兰大学澳大利亚生物工程和纳米技术研究所在内的多家研究机构合作开展的,由昆士兰大学临床研究中心教授Nicholas Fisk领导。
间充质干细胞(mesenchymal stem cells, MSCs)能够被用来修复骨骼和潜在性地修复其他器官。这项研究揭示一种生产间充质干细胞的新方法。
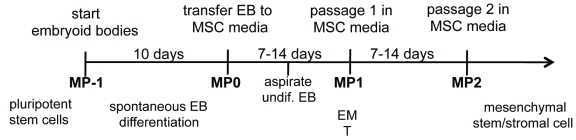
Fisk教授说,“我们使用一种小分子SB431542---一种转化生长因子β (transforming growth factor-β, TGF-β)途径抑制剂---诱导胚胎干细胞10天(就可产生间充质干细胞),产生速度要比文献中报道的其他研究快得多。这种技术也可适用于较少引起争议的诱导性多功能干细胞(induced pluripotent stem cell, iPSC)。”
“为了使得多能性成熟干细胞能够应用于临床,在注射到受损器官之前,它们必须收到告诉它们需要变成哪些细胞类型的信号,否则它们可能形成肿瘤。”
“因为只有少量间充质干细胞存在于骨髓中而且从健康供者中收集骨髓的方法是侵入式的,所以在实验室中能够大量制造我们自己的间充质干细胞是未来间充质干细胞大规模用于临床治疗的一次激动人心的进步。”
“我们能够证实这些新形式的干细胞表现出骨髓干细胞的所有特征,而且我们当前正在研究它们的骨修复能力。”
澳大利亚生物工程和纳米技术研究所副教授和该研究项目的共同研究员Ernst Wolvetang说,就基于干细胞的疗法的医学转化而言,这种新方法已克服了一道重要的障碍。
Wolvetang副教授说,“我们对这项研究感到非常激动。”
这项研究成果发表在2012年2月份那期《干细胞转化医学》期刊上。
Small Molecule Mesengenic Induction of Human Induced Pluripotent Stem Cells to Generate Mesenchymal Stem/Stromal Cells
Yen Shun Chen, Rebecca A. Pelekanos, Rebecca L. Ellis, Rachel Horne, Ernst J. Wolvetang and Nicholas M. Fisk
The translational potential of mesenchymal stem/stromal cells (MSCs) is limited by their rarity in somatic organs, heterogeneity, and need for harvest by invasive procedures. Induced pluripotent stem cells (iPSCs) could be an advantageous source of MSCs, but attempts to derive MSCs from pluripotent cells have required cumbersome or untranslatable techniques, such as coculture, physical manipulation, sorting, or viral transduction. We devised a single-step method to direct mesengenic differentiation of human embryonic stem cells (ESCs) and iPSCs using a small molecule inhibitor. First, epithelial-like monolayer cells were generated by culturing ESCs/iPSCs in serum-free medium containing the transforming growth factor-β pathway inhibitor SB431542. After 10 days, iPSCs showed upregulation of mesodermal genes (MSX2, NCAM, HOXA2) and downregulation of pluripotency genes (OCT4, LEFTY1/2). Differentiation was then completed by transferring cells into conventional MSC medium. The resultant development of MSC-like morphology was associated with increased expression of genes, reflecting epithelial-to-mesenchymal transition. Both ESC- and iPSC-derived MSCs exhibited a typical MSC immunophenotype, expressed high levels of vimentin and N-cadherin, and lacked expression of pluripotency markers at the protein level. Robust osteogenic and chondrogenic differentiation was induced in vitro in ES-MSCs and iPS-MSCs, whereas adipogenic differentiation was limited, as reported for primitive fetal MSCs and ES-MSCs derived by other methods. We conclude that treatment with SB431542 in two-dimensional cultures followed by culture-induced epithelial-to-mesenchymal transition leads to rapid and uniform MSC conversion of human pluripotent cells without the need for embryoid body formation or feeder cell coculture, providing a robust, clinically applicable, and efficient system for generating MSCs from human iPSCs.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言









#成体干细胞#
60
#生产#
54