抗骨髓瘤新药前景
2012-06-19 田磊磊 医学论坛网
一项小型Ⅰ期研究的研究者报告,实验性口服蛋白酶体抑制剂MLN9708可能有望用于治疗复发或对治疗耐药的多发性骨髓瘤患者。 58例多发性骨髓瘤患者中有6例至少得到客观缓解,其中1例接近完全缓解,美国埃默里大学Winship肿瘤研究所的Sagar Loniol博士说,这例患者既往未接受蛋白酶体抑制剂治疗,并

Methods: Pts aged ≥18 yrs with measurable MM received MLN9708 on d 1, 4, 8, and 11 of 21-d cycles. In the dose-escalation phase, pts required ≥2 prior therapies (including bortezomib, thalidomide/lenalidomide, and corticosteroids). At the MTD (2.0 mg/m2), pts were enrolled to relapsed and refractory [RR], bortezomib-relapsed [VR], proteasome-inhibitor [PI] naïve, and carfilzomib [CZ] expansion cohorts.
Results: 57 pts (53% M) were enrolled, 37 to the expansion cohorts (16 RR, 14 VR, 6 PI naïve, 1 CZ). Median age was 65 yrs (range 50-86). Median number of prior lines of therapy was 4 (range 1-28); 88%, 84%, 61%, and 5% had prior bortezomib, lenalidomide, thalidomide, and carfilzomib, respectively. Pts have received a median of 3 cycles (range 1-24) to date (data cut-off Dec 1, 2011); 7 (12%) have received ≥13 cycles. Drug-related AEs were seen in 89% of pts, including fatigue (46%), thrombocytopenia (40%), and nausea (30%); 63% had drug-related grade ≥3 AEs, including thrombocytopenia (33%), neutropenia (14%), fatigue (9%), and rash (7%). Only 6 (11%) pts had drug-related peripheral neuropathy (PN; no grade ≥3). 7 pts discontinued due to AEs. 2 pts died on study, due to PD and an unrelated cardiac disorder. Of 46 response-evaluable pts, 6 have achieved ≥PR, with 1 sCR (PI naïve cohort) and 5 PRs (2 in dose-escalation, 1 in RR, 2 in VR cohorts), and 1 VR pt has achieved MR, with duration of disease control of up to 18.6 mo. PK analyses showed MLN2238 (biologically active hydrolysis product) has linear plasma PK (0.8-2.23 mg/m2), Tmax of 0.5-1.25 hr, and terminal half-life of 4-6 d. A dose-dependent increase in whole blood 20S proteasome inhibition was observed.
Conclusions: Current data suggest MLN9708 has clinical activity in heavily pretreated MM pts, with durable responses/disease control, and is generally well tolerated with infrequent low-grade PN.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言
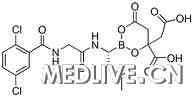










如果有静脉用蛋白酶体抑制剂将是个好事情
155