PNAS:改善睡眠药物可能导致新的癌症疗法
2012-05-28 Beyond 生物谷
近日,一项刊登在PNAS杂志上的最近研究发现,一种廉价的治疗睡眠障碍的药物似乎也是一种癌细胞的强效抑制剂。Fred Hutchinson癌症研究中心的研究人员通过迅速分析基因组后发现了这一药物的作用靶点,研究人员表示这项研究对更有效和更安全的治疗癌症的发展具有深远的影响。 研究主要领导者——Carla Grandori医学博士使用高通量筛选技术结合siRNA基因沉默技术发现了癌细胞的致命弱点,这
近日,一项刊登在PNAS杂志上的最近研究发现,一种廉价的治疗睡眠障碍的药物似乎也是一种癌细胞的强效抑制剂。Fred Hutchinson癌症研究中心的研究人员通过迅速分析基因组后发现了这一药物的作用靶点,研究人员表示这项研究对更有效和更安全的治疗癌症的发展具有深远的影响。
研究主要领导者——Carla Grandori医学博士使用高通量筛选技术结合siRNA基因沉默技术发现了癌细胞的致命弱点,这一致命弱点是由Myc基因驱动的。
幸运的是,Myc驱动癌细胞有一个致命的弱点。其快速生长和分裂会破坏它们的DNA,肿瘤细胞依赖于其他基因修复损害。一旦抑制这些基因可以就削弱癌细胞的能增长力。
Grandori和他的团队发现了超过100个基因能杀死Myc驱动的癌细胞,而不影响正常细胞,这意味着这些基因可能是一个新的、无毒的癌症治疗的靶标。
其中一个特别有前途的基因就是CSNK 1,该基因在杀死癌细胞的同时并不影响正常组织,而且已经有该基因的抑制剂诞生,该抑制剂是一种化合物,最初开发用来调节睡眠周期的。
高通量筛选技术结合siRNA沉默技术,Grandori表示:癌症治疗方可能会出现一个根本性的转变。

doi:10.1073/pnas.1121119109
PMC:
PMID:
Functional genomics identifies therapeutic targets for MYC-driven cancer
Masafumi Toyoshima, Heather L. Howie, Maki Imakura, Ryan M. Walsh, James E. Annis, Aaron N. Chang, Jason Frazier, B. Nelson Chau, Andrey Loboda, Peter S. Linsley, Michele A. Cleary, Julie R. Park, and Carla Grandori
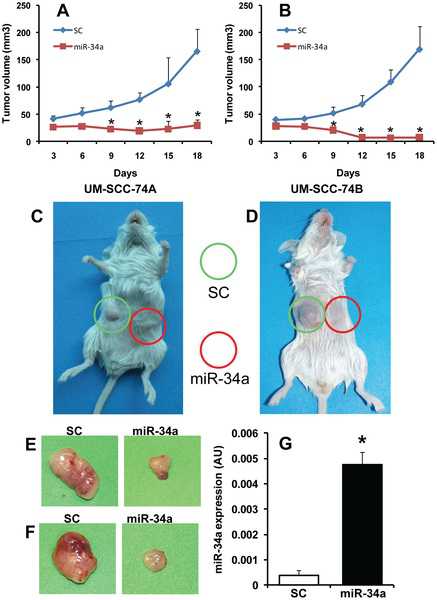
MYC oncogene family members are broadly implicated in human cancers, yet are considered “undruggable” as they encode transcription factors. MYC also carries out essential functions in proliferative tissues, suggesting that its inhibition could cause severe side effects. We elected to identify synthetic lethal interactions with c-MYC overexpression (MYC-SL) in a collection of ∼3,300 druggable genes, using high-throughput siRNA screening. Of 49 genes selected for follow-up, 48 were confirmed by independent retesting and approximately one-third selectively induced accumulation of DNA damage, consistent with enrichment in DNA-repair genes by functional annotation. In addition, genes involved in histone acetylation and transcriptional elongation, such as TRRAP and BRD4, were identified, indicating that the screen revealed known MYC-associated pathways. For in vivo validation we selected CSNK1e, a kinase whose expression correlated with MYCN amplification in neuroblastoma (an established MYC-driven cancer). Using RNAi and available small-molecule inhibitors, we confirmed that inhibition of CSNK1e halted growth of MYCN-amplified neuroblastoma xenografts. CSNK1e had previously been implicated in the regulation of developmental pathways and circadian rhythms, whereas our data provide a previously unknown link with oncogenic MYC. Furthermore, expression of CSNK1e correlated with c-MYC and its transcriptional signature in other human cancers, indicating potential broad therapeutic implications of targeting CSNK1e function. In summary, through a functional genomics approach, pathways essential in the context of oncogenic MYC but not to normal cells were identified, thus revealing a rich therapeutic space linked to a previously “undruggable” oncogene.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言












#PNAS#
78
#癌症疗法#
68