2017年FDA批准新药名单,上半年已达21种!
2017-05-25 诸平 诸平
据FDA官网(http://www.fda.org)2017年5月22日提供的最新公布结果,截止2017年5月22日,FDA已经批准了21种新药,约占2016年FDA批准新药数量(22种)的95%,去年创造新药新低,今 年有望获得重大突破,甚至能创造近年来审批新药的新高。2011-2017年5月5日FDA批准新药数量变化见图1所示。 图1 FDA2011-2017年批准新药数量变
据FDA官网(http://www.fda.org)2017年5月22日提供的最新公布结果,截止2017年5月22日,FDA已经批准了21种新药,约占2016年FDA批准新药数量(22种)的95%,去年创造新药新低,今 年有望获得重大突破,甚至能创造近年来审批新药的新高。2011-2017年5月5日FDA批准新药数量图。
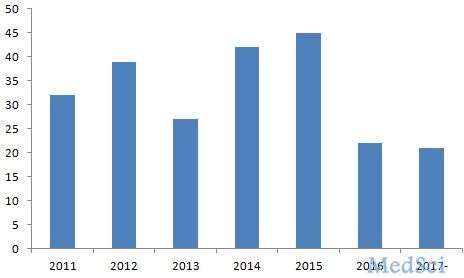
图1 FDA2011-2017年批准新药数量变化
2017年以来(截止2017年5月22日)FDA批准新药名单。
表1 2017年FDA批准新药一览表
| No. | Drug Name | Active Ingredient | Approval Date | FDA-approved use on approval date | News |
|---|---|---|---|---|---|
| 21. | Kevzara | sarilumab | 5/22/2017 | To treat adult rheumatoid arthritis | Link |
| 20. | Radicava | edaravone | 5/5/2017 | To treat patients with amyotrophic lateral sclerosis (ALS) Press Release | Link |
| 19. | Imfinzi | durvalumab | 5/1/2017 | To treat patients with locally advanced or metastatic urothelial carcinoma Web Post Drug Trials Snapshot | Link |
| 18. | Tymlos | abaloparatide | 4/28/2017 | To treat osteoporosis in postmenopausal women at high risk of fracture or those who have failed other therapies | Link |
| 17. | Rydapt | midostaurin | 4/28/2017 | To treat acute myeloid leukemia Press Release Drug Trials Snapshot | Link |
| 16. | Alunbrig | brigatinib | 4/28/2017 | To treat patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Drug Trials Snapshot | Link |
| 15. | Brineura | cerliponase alfa | 4/27/2017 | To treat a specific form of Batten disease Press Release | Link |
| 14. | Ingrezza | valbenazine | 4/11/2017 | To treat adults with tardive dyskinesia Press Release Drug Trials Snapshot | Link |
| 13. | Austedo | deutetrabenazine | 4/3/2017 | For the treatment of chorea associated with Huntington’s disease Drug Trials Snapshot | Link |
| 12. | Ocrevus | ocrelizumab | 3/28/2017 | To treat patients with relapsing and primary progressive forms of multiple sclerosis Press Release Drug Trials Snapshot | Link |
| 11. | Dupixent | dupilumab | 3/28/2017 | To treat adults with moderate-to-severe eczema (atopic dermatitis) Press Release Drug Trials Snapshot | Link |
| 10. | Zejula | niraparib | 3/27/2017 | For the maintenance treatment for recurrent epithelial ovarian, fallopian tube or primary peritoneal cancers Press Release Drug Trials Snapshot | Link |
| 9. | Symproic | naldemedine | 3/23/2017 | For the treatment of opioid-induced constipation | Link |
| 8. | Bavencio | avelumab | 3/23/2017 | To treat metastatic Merkel cell carcinoma Press Release Drug Trials Snapshot | Link |
| 7. | Xadago | safinamide | 3/21/2017 | To treat Parkinson’s disease Press Release Drug Trials Snapshot | Link |
| 6. | Kisqali | ribociclib | 3/13/2017 | To treat postmenopausal women with a type of advanced breast cancer Drug Trials Snapshot | Link |
| 5. | Xermelo | telotristat ethyl | 2/28/2017 | To treat carcinoid syndrome diarrhea Press Release Drug Trials Snapshot | Link |
| 4. | Siliq | brodalumab | 2/15/2017 | To treat adults with moderate-to-severe plaque psoriasis Press Release Drug Trials Snapshot | Link |
| 3. | Emflaza | deflazacort | 2/9/2017 | To treat patients age 5 years and older with Duchenne muscular dystrophy (DMD) Press Release Drug Trials Snapshot | Link |
| 2. | Parsabiv | etelcalcetide | 2/8/2017 | To treat secondary hyperparathyroidism in adult patients with chronic kidney disease undergoing dialysis Drug Trials Snapshot | Link |
| 1. | Trulance | plecanatide | 1/19/2017 | To treat Chronic Idiopathic Constipation (CIC) in adult patients. Press Release Drug Trials Snapshot | Link |
* This information is currently accurate. In rare instances, it may be necessary for FDA to change a drug’s new molecular entity (NME) designation or the status of its application as a novel new biologics license application (BLA). For instance, new information may become available which could lead to a reconsideration of the original designation or status. If changes must be made to a drug’s designation or the status of an application as a novel BLA, the Agency intends to communicate the nature of, and the reason for, any revisions as appropriate.
更多更新信息请浏览FDA官网:详细请点击进入
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言










#FDA批准#
34
学习了,谢谢分享
93
哇,又有这么多新药出来了
89
非常好的文章,学习了,很受益
80
单抗类好多
95
很好,学习了
76
可以用更多更好的药了。
45
按这速度和往年对比,虽有比去年提高,但是要超过2015年恐怕还是有难度。
49
阅读了谢谢分享
34
谢谢分享
54