AAN 2013:重症肌无力治疗新药研究
2013-04-17 AAN dxy
一项发表于美国第65届神经病学会年会的初步数据表明,肌萎缩脊髓侧索硬化(ALS)研究用药物tirasemtiv(Cytokinetics公司生产)可能对重症肌无力也有一定作用。研究者美国杜克大学重症肌无力诊所创始人,北卡罗来纳州达勒姆Donald B. Sanders博士说,“这些结果表明tirasemtiv可改善重症肌无力的功能,且这些研究结果将被用于进一步支持tirasemtiv在神经肌肉疾病
一项发表于美国第65届神经病学会年会的初步数据表明,肌萎缩脊髓侧索硬化(ALS)研究用药物tirasemtiv(Cytokinetics公司生产)可能对重症肌无力也有一定作用。
研究者美国杜克大学重症肌无力诊所创始人,北卡罗来纳州达勒姆Donald B. Sanders博士说,“这些结果表明tirasemtiv可改善重症肌无力的功能,且这些研究结果将被用于进一步支持tirasemtiv在神经肌肉疾病中的应用。几乎所有用于治疗重症肌无力的药物如强的松,硫唑嘌呤等都对免疫功能有影响。但tirasemtiv是对症疗法,可以改善对其它治疗无应答患者或有禁忌患者的肌力。”
据说该药物是通过增加对钙的敏感性选择性激活快骨骼肌肌钙蛋白复合物,从而增加骨骼肌力对神经元输入的应答,并延缓发作,减少肌肉疲劳程度。Sanders博士指出,已证明单剂量能增加健康志愿者,ALS患者以及小腿跛行患者骨骼肌强度和耐力。Tirasemtiv可减少重症肌无力大鼠模型肌肉易疲劳,增加肌肉力量和握力。
研究概述
目前的研究是一项双盲,随机,3周期交叉设计研究,共包括32例全身性重症肌无力患者,定量重症肌无力(QMG)分级评分为2或3分。上述患者随机给予单次口服剂量的安慰剂,250mg的tirasemtiv和500mg的tirasemtiv,间隔时间约为1周。这项试验旨在评估tirasemtiv对各种骨骼肌强度以及乏力检测的影响,包括肺功能的检测。服药后6小时,观察该药物QMG评分剂量相关改善。
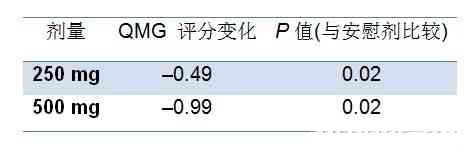
表1 Tirasemtiv与安慰剂的QMG分数变化
分析显示服用500mg tirasemtiv5小时后QMG评分提高3分以上的患者是安慰剂组的两倍(12:6,P = 0.098)。此外,预测肺活量(FVC)百分比较安慰剂组高。
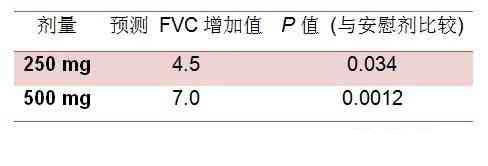
Table 2. Increase in Predicted FVC vs Placebo
研究报道该药物耐受性良好,无严重不良事件发生。在本试验中最常报告的不良事件是头晕,所有均较轻,1例为中度。
另一项有关Tirasemtiv治疗肌萎缩侧索硬化的400例患者2b期临床试验正在进行中。
与肌无力相关的拓展阅读:
- 进行性肌无力伴肌萎缩 更多信息请点击:有关肌无力更多资讯
Tirasemtiv: Initial Promise in Myasthenia Gravis.medscape
San Diego, California — The investigational drug tirasemtiv (Cytokinetics), which is being studied for the first indication of amyotrophic lateral sclerosis (ALS), may also have a role in myasthenia gravis, preliminary data suggest.
Presenting the initial data in patients with myasthenia gravis, Donald B. Sanders, MD, founder of the Duke Myasthenia Gravis Clinic, Durham, North Carolina, said, "These results suggest that tirasemtiv improves function in myasthenia gravis and they will be used to support further development of tirasemtiv in neuromuscular diseases."
"Almost all drugs used in myasthenia gravis are immunologics, such as prednisolone, azathioprine etc. But tirasemtiv is a symptomatic therapy could improve strength in patients who don't respond to other treatments or in whom they are contraindicated," Dr. Sanders commented to Medscape Medical News.
He added, "This was a pilot study to see if we could find a signal of efficacy. We did see such a signal. It could be particularly valuable in the subgroup of patients who have congenital myasthenic syndromes for whom there are few therapies available. It could also have a role in other neuromuscular diseases that cause weakness."
The results were presented here at the recent American Academy of Neurology (AAN) 65th Annual Meeting.
Activates Skeletal Muscle Troponin
The drug is said to selectively activate the fast skeletal muscle troponin complex by increasing its sensitivity to calcium, thereby increasing skeletal muscle force in response to neuronal input and delaying the onset and reducing the degree of muscle fatigue.
Dr. Sanders noted that single doses have been shown to increase skeletal muscle strength and endurance in healthy volunteers, in patients with ALS, and in patients with calf claudication. In a passive transfer rat model of myasthenia gravis, tirasemtiv decreased muscle fatigability and increased muscle force and grip strength.
In the current study, which had a double-blind, randomized, 3-period crossover design, 32 patients with generalized myasthenia gravis with a Quantitative Myasthenia Gravis (QMG) grade of 2 or 3 in 2 or more muscles received single oral doses of placebo, 250 mg of tirasemtiv, and 500 mg of tirasemtiv in random order and approximately 1 week apart.
The main objectives of this trial were to assess the effects of tirasemtiv on various measures of skeletal muscle strength and fatigue, including measures of pulmonary function.
At 6 hours after dosing, dose-related improvements in the QMG score (a validated index of disease severity) were seen with the drug.

In a responder analysis, twice as many patients improved by 3 points or more 6 hours after dosing with 500 mg of tirasemtiv compared with 6 hours after placebo (12 vs 6; P = .098). In addition, the percentage predicted forced vital capacity (FVC) increased relative to placebo.

The drug was said to be well tolerated, with no serious adverse events occurring. The most commonly reported adverse event in this trial was dizziness, which was mild in all but 1 case that was classified as moderate.
Tirasemtiv is being developed first for ALS, for which it is being investigated in a phase 2b study in 400 patients.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言








#肌无力#
68
#AAN#
74